
Apple Gets FDA Approval for New Apple Watch ECG Feature Ahead of WWDC

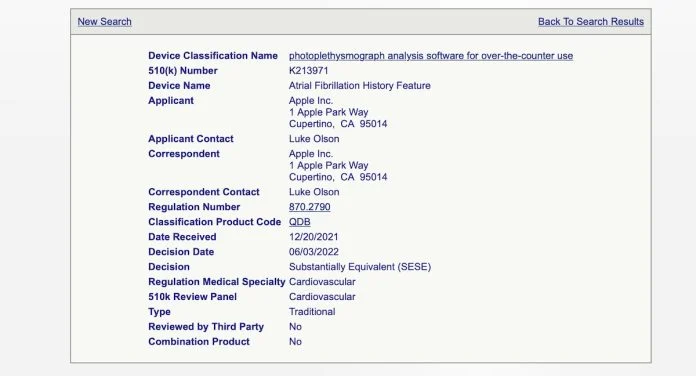
According to a report from MyHealthyApple, Apple has received FDA 510(k) approval for a new Apple Watch feature designed to keep track of a user’s Atrial Fibrillation (AFib) history.
Judging by the approval filing, the feature will allow users to check their AFib history on demand.
Atrial Fibrillation is when the heart beats at an irregular rhythm. While AFib is one of the most common arrhythmias a person can experience, it can lead to an increased risk of stroke, heart failure, and other cardiovascular complications.
Apple introduced electrocardiogram (ECG) technology with the Apple Watch Series 4 back in 2018. Apple wasn’t able to launch ECG tracking in Canada right away, but the feature eventually landed in the Great White North back in July 2019.
Version 1 of Apple’s ECG app for watchOS can check for AFib between 50 and 120 BPM (beats per minute). Version 2 of the ECG app can check for AFib between 50 and 150 BPM.
According to Apple, the company’s ECG app was proven to be able to accurately classify an ECG recording into AFib and sinus rhythm in a clinical trial comprising around 600 subjects. The results of the trial indicated that the ECG app could successfully classify AFib with 98.3% specificity, and sinus rhythm with 99.6% specificity.
While FDA approval says nothing about when the feature will start rolling out, it will likely be part of the next iteration of watchOS for the Apple Watch. Apple is expected to show off watchOS 9 at this year’s Worldwide Developer Conference (WWDC) later today.

